GB/T 14710-2009 'Environmental Requirements and Test Methods for Medical Electrical Appliances' was proposed by the National Food and Drug Administration, updated and released on November 15, 2009, and officially implemented on May 1, 2010. The purpose of the standard is to evaluate the adaptability of medical electrical equipment in various working environments and simulated storage and transportation environments by applying certain environmental stresses.
Is it necessary to refer to the GB/T 14710-2009 standard when environmental testing is required for medical electrical equipment?
The answer from the Medical Device Technical Evaluation Center of the National Drug Administration is as follows:
If the mandatory standards applicable to the products to be declared comply with the GB/T 14710-2009 standard, testing should be carried out in accordance with the GB/T 14710-2009 standard and the relevant requirements of the mandatory standard.
Interpretation: If the mandatory standards applicable to the product have clear guidelines, guiding principles, etc., as long as these documents have clear requirements to comply with the GB/T 14710 standard, they must be implemented according to the requirements;
If the product to be declared is used in special environments (such as high temperature, high humidity, or low temperature), supporting information that the product can be used in the corresponding environment should be provided in the research materials.
Interpretation: If all mandatory standards (including recommended standards and guiding principles) applicable to the product do not require the application of the GB/T 14710 standard, then it is also necessary to consider whether the registered and declared product will be used in special environments (such as high temperature, low temperature, high humidity, or vibration). If it is confirmed that it will be used, then it is still necessary to provide supporting information that the product can be used in that environment, The test report passed the environmental test verification in accordance with GB/T 14710-2009.
Standard Overview:
This standard specifies the purpose, environmental grouping, transportation testing, adaptability to power supply, reference test conditions, special circumstances, test procedures, test sequence, test requirements, test methods, and detailed rules that should be specified when referencing this standard for environmental testing of medical electrical equipment.
Applicable scope of the standard:
Applicable to all electrical equipment or systems that meet the definition of medical devices.
The following content will summarize the content of the GB/T 14710-2009 standard and summarize the environmental testing requirements and methods for medical electrical equipment:
2. Environmental grouping of medical electrical equipment:
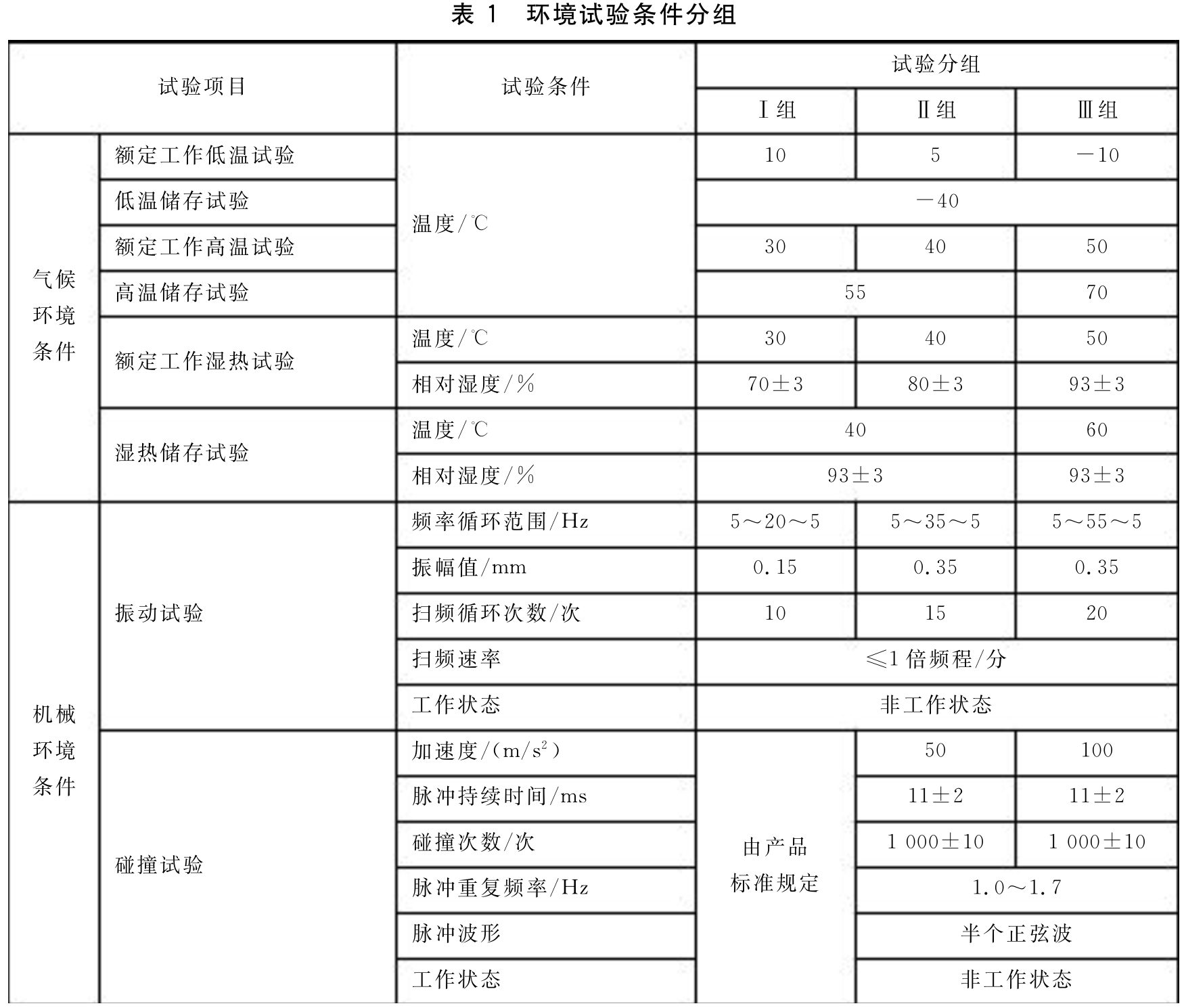
The equipment is divided into three groups based on climatic and mechanical environments, as follows:
2.1 Climate and environmental grouping (according to usage conditions):
a) Group I
Use in a good environment. Usually refers to the use of equipment in a controlled environment with air conditioning and other equipment;
b) Group II
Used in general environments. Usually refers to the use of equipment in an environment with optimal supply and ventilation;
c) Group III
Used in harsh environments. Usually refers to the use of equipment in non insulated and ventilated environments, as well as similar outdoor environments.
2.2 Mechanical environment grouping (based on transportation and circulation conditions):
a) Group I
Equipment that is carefully operated and subjected to slight vibrations and impacts during transportation and circulation generally refers to fixed and rarely moved equipment;
b) Group II
Equipment that is allowed to be subjected to general vibration and impact during use, generally referring to equipment that is easy to move;
c) Group III
Equipment that is subject to vibration and impact during frequent transportation, loading and unloading, and handling.
3. Environmental condition grouping:
3. Requirements for finished product packaging and transportation test
Under normal factory packaging conditions, the equipment should be tied to the rear of the truck in the marked 'up' position. During the test, the load capacity of the truck should be 1/3 of its rated load capacity.
Road surface: third-class highway according to JTG B01-2003 standard.
Driving distance: 200 km.
Driving speed: 30 km/h~40 km/h
According to the above requirements, the test can be conducted using a truck or a transportation test device. After the test is completed, inspect the fasteners of the equipment for looseness and conduct testing according to the inspection items specified in the product standard.
4. Adaptability to power supply
4.1 For equipment powered by the power grid, the test voltage shall be 110% or 90% of the rated value.
4.2 For equipment with special requirements for power frequency and voltage, the working range and test method of frequency and voltage can be separately specified in the product standard.
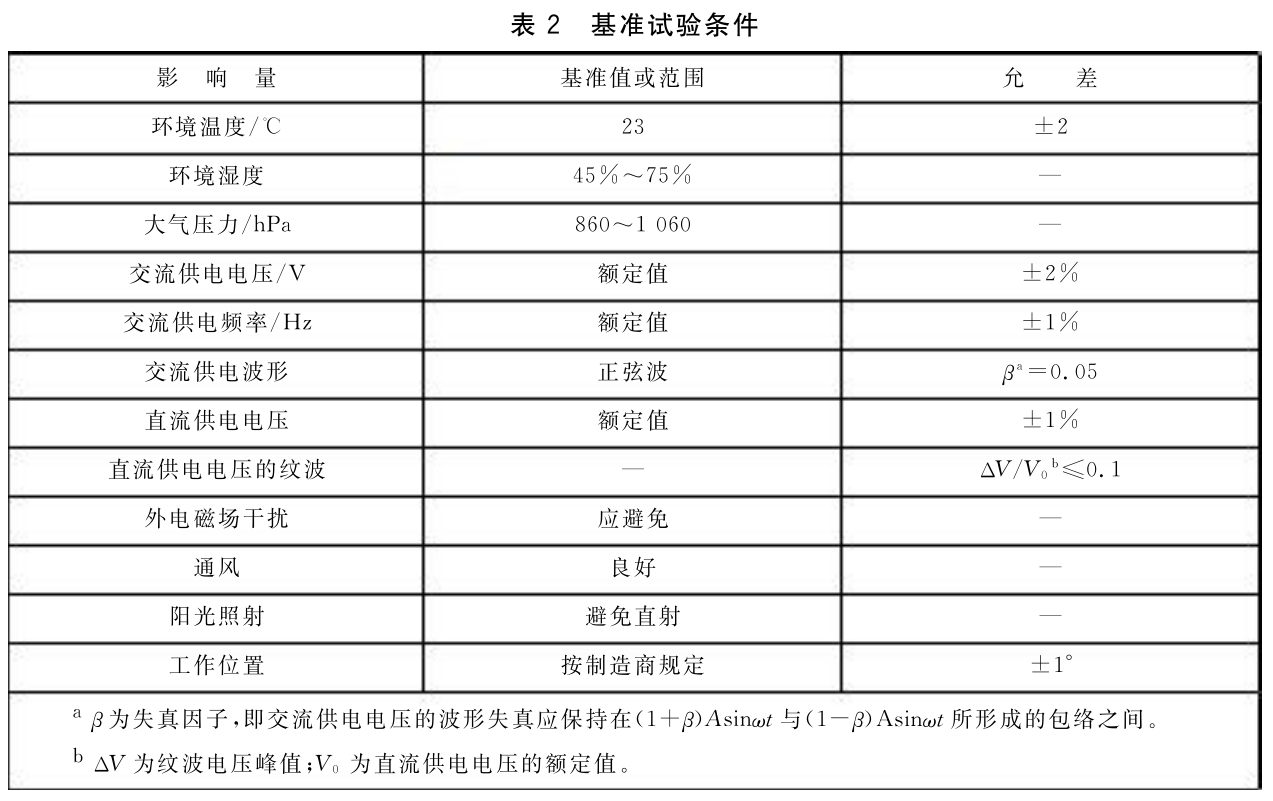
5. Reference test conditions
The benchmark test conditions, benchmark values or ranges, and tolerances are shown in Table 2.
When there is no doubt, it can be carried out under the conditions of+10 ℃ -+40 ℃, relative humidity of 30% -75%, AC voltage of ± 10% of rated value, and power frequency of ± 2% of rated value.
Conduct the experiment in the following order:
a) Rated working low temperature test;
b) Low temperature storage test;
c) Rated working high temperature test;
d) High temperature storage test;
e) Rated working damp heat test;
f) Damp heat storage test;
g) Vibration test;
h) Collision test;
i) Transportation test.
If the test sequence is affected, it shall be specified by the product standard